
SCIENCE COOKIES
Science articles with chocolate chips
Artificial intelligence solves the Schrödinger equation
Published: 18/1/2021
Author: Andrea Garma
Jan Hermann, Zeno Schätzle and Frank Noé, researchers at the Freire Universität in Berlin, have made important discoveries and conclusions by using quantum chemistry to analytically conclude that Shrödinger's electronic equation can only be solved by the hydrogen atom. Quantum chemistry is intended to give a prediction of chemical and physical properties based on the presence of its atoms in space, the above to avoid high resource consumption.
It was not until the research team's experiment in Berlin that the first exact solution to the equation was found, which had previously been impossible. With this solution, new opportunities are opened in the world of chemical science.
The wave function is a representation of the behavior of the electrons in a molecule, it depends on a large number of variables, which makes it difficult to specify this function, and in turn, infers inaccuracy in the results, thus limiting the fundamental prediction of the chemical methods. Both the Shrödinger equation and quantum chemistry depend on the wave function parameter.
Artificial intelligence
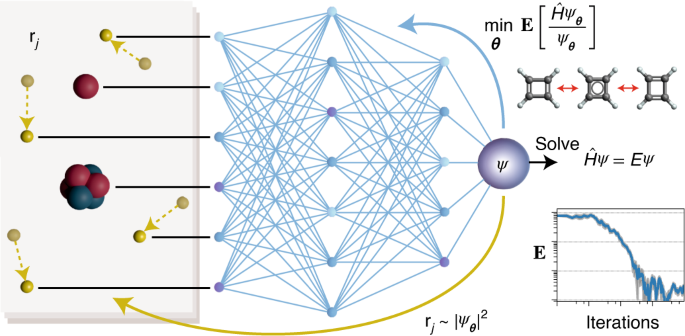
Hermann, Schätzle and Noé decided to develop a deep learning method, which makes it possible to obtain an almost perfect combination at the computational level. The computer's neural network can represent wave functions of electrons. Instead of taking a standard approach of simple mathematical components, we opted for a network that learned the more complex patterns of electron placement around nuclei.
Keep feeding your brain more Science Cookies!
References
https://www.abc.es/ciencia/abci-consigue-resolver-ecuacion-schrodinger-202012292032_noticia.html





